Polar Speed Distribution Ltd (UK) has selected 4S eLog to optimise its management of their temperature controlled, pharmaceutical distribution warehouses throughout the UK.
Pharmaceutical manufacturing software
The pharmaceutical industry involves the development, production, testing and sale of drugs licensed for medical use. Pharmaceutical companies are required to abide by a number of laws and regulations regarding patents and tests etc. in order to ensure the efficacy and safety of their products. The process of manufacturing and distributing pharmaceutical products requires the purchase of raw materials for bulk synthesis of active and inactive ingredients. Products flow through manufacturers’ warehouses, wholesale distributors/3rd party logistics providers, retail pharmacies, medical institutions, and finally to the patient. Some products make their way back to their manufacturers due to recalls and returns.
Winners of the Supply Chain Distinction Awards 2009
Jun 18, 2009

Winners of the Supply Chain Distinction Awards 2009 are revealed as the supply chain elite gathered in Germany
Eastman Chemical implements the CA Clarity system
Jun 15, 2009
Eastmans IT project office reviewed 32 potential software solutions and chose Clarity.
VoiteQ launches VoiceMan Velocity to improve forklift truck efficiency
Jun 03, 2009
Developed to optimise forklift truck operated tasks, VoiceMan Velocity allows forklift truck operatives to carry out just in-time replenishments, therefore ensuring high stock availability.
Improving the effectiveness of traditional demand planning systems
Jun 03, 2009

This white paper from Infosys discusses the limitations of the existing Demand Planning Systems in terms of forecasting methods, statistical tools, dashboard and decision support tools.
Approach to Effective APO / DP Design and Implementation
Jun 03, 2009

This Infosys white paper demonstrates how an efficient and effective SAP Advanced Planner and Optimizer (APO) Demand Planning (DP) design, ensures the long-term sustainability of the solution, and provides an overview of the key features and functionalities in APO DP.
Easy creation of identification cards
Jun 02, 2009
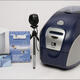
Zebra Technologies unveils QuikCard ID solution for fast, easy creation of identification cards.
UHF RFID passive transponder technology makes another leap
May 28, 2009
Trolley Scan has announced the development of a new passive transponder that is claimed to have approximately 100% improvement in range over previous versions.
Software firms remain positive about Europe
May 07, 2009

Senior executives in internationally dynamic software firms say that the current economic crisis will not significantly affect their European business models.
Transportation and logistics industry has most successful extra-marital relations
May 05, 2009

Those working in the transportation and logistics industry are more likely to manage an affair than other professionals
Pharmaceutical Manufacturing Software
Pharmaceutical manufacturing software is a type of software that is used to manage the manufacturing of pharmaceutical products. It typically includes modules for managing formulation, production planning, quality control, and compliance.
Some of the common features of pharmaceutical manufacturing software include:
- Formulation management: This module allows users to create and manage formulations for pharmaceutical products. It typically includes features for managing ingredients, dosages, and manufacturing steps.
- Production planning: This module allows users to plan and schedule the production of pharmaceutical products. It typically includes features for managing capacity, demand, and resources.
- Quality control: This module allows users to manage the quality control of pharmaceutical products. It typically includes features for managing testing, inspection, and data analysis.
- Compliance: This module allows users to manage the compliance of pharmaceutical products with regulations. It typically includes features for managing documentation, auditing, and training.
Some of the benefits of using pharmaceutical manufacturing software include:
- Improved efficiency: Pharmaceutical manufacturing software can help to improve the efficiency of the manufacturing process by automating tasks, streamlining workflows, and providing real-time data.
- Increased quality: Pharmaceutical manufacturing software can help to increase the quality of pharmaceutical products by providing a more rigorous and consistent approach to quality control.
- Enhanced compliance: Pharmaceutical manufacturing software can help to enhance compliance with regulations by providing a centralized repository for documentation and by automating auditing processes.
Some of the challenges of using pharmaceutical manufacturing software include:
- Cost: Pharmaceutical manufacturing software can be expensive to purchase and implement.
- Complexity: Pharmaceutical manufacturing software can be complex to use, especially for organizations that are not familiar with the software.
- Data security: Pharmaceutical manufacturing software typically handles sensitive data, so it is important to ensure that the software is secure.

